November 14, 2025 – For the first time, bovine cells can naturally become immortal—providing a safe, stable, and scalable source of cells for affordable, cultivated beef production and removing one of the biggest technical and regulatory barriers to producing affordable cultivated beef, according to a new study conducted by researchers at Hebrew University of Jerusalem (HU).
Stable, self-renewing cell lines are the foundation of any large-scale cell culture system, just as yeast or bacterial strains underpin pharmaceutical and food manufacturing. As part of its longstanding focus on reshaping the future of sustainable food, in this study, Hebrew University and Believer Meats (an Israeli food-tech startup founded by Prof. Yaakov Nahmias from HU’s Alexander Grass Center for Bioengineering, who led this study), overturn the long-held assumption that bovine cells could only be immortalized through gene editing and that such processes were not possible in large mammals due to their natural resistance to cellular transformation. The researchers have unlocked a natural pathway to immortalize bovine cells, finding that these cells can spontaneously renew themselves indefinitely without the need for genetic modification.
This breakthrough is a potential game-changer for creating sustainable, ethical meat without the environmental toll of traditional livestock farming, and demonstrates a natural and safe route to establish these cell lines in cattle, suggesting that price parity with conventional beef could theoretically be reached using continuous cell-based manufacturing—bringing cultivated meat closer to mainstream viability.
“Several years ago, we showed chicken cells immortalized without such interventions, but the consensus in the field was that bovine cells could not do the same,” said Prof. Nahmias. “What worked relatively quickly in chickens became an exhaustive pursuit in bovine cells. We had to continuously culture bovine cells for more than 18 months before the first self-renewing colonies emerged.”
Breaking Nature’s Code
In traditional cell biology, animal cells stop dividing after a certain number of generations and enter a state known as senescence. Until now, cattle cells could only be induced to bypass this limit by disabling genes involved in cell cycle regulation, raising regulatory and safety concerns.
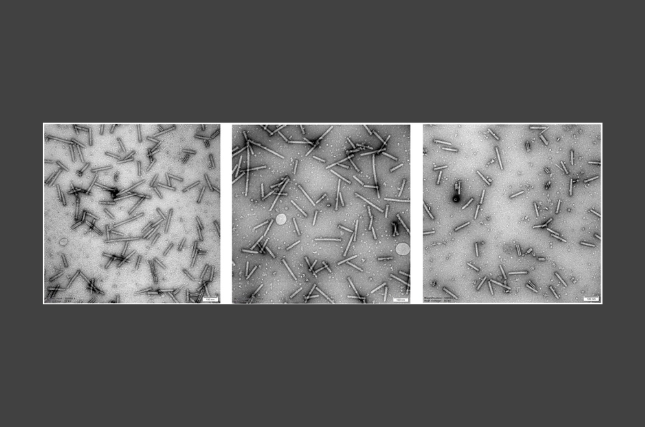
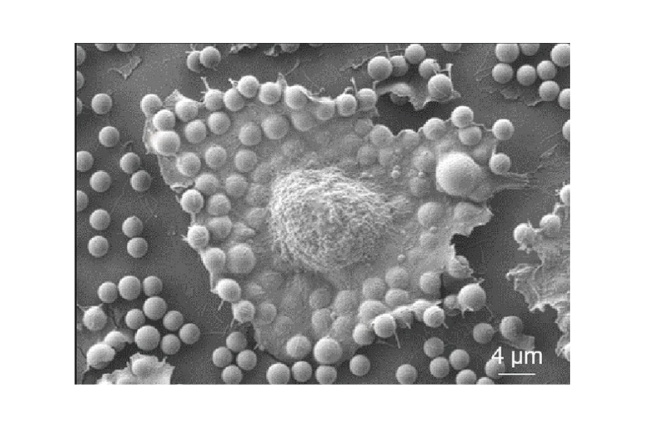
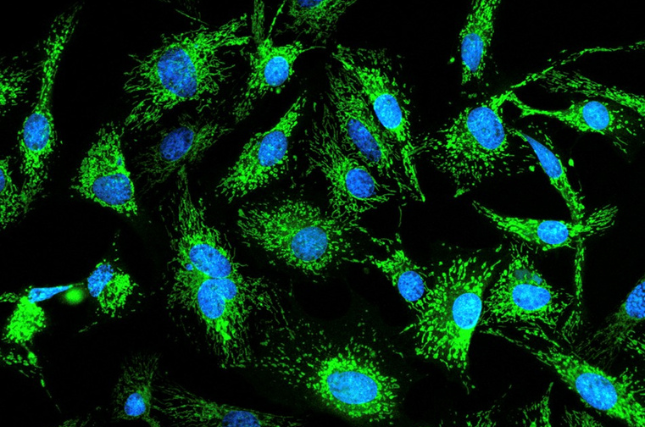
The researchers isolated cells from both Holstein and Simmental cows and grew them in the laboratory for over 500 days, tracking their progression through aging and senescence up to 180 days of culture. Despite months of apparent inactivity, the team persisted—and after 240 cell generations, spontaneously renewing bovine cells emerged. Molecular analysis revealed that the process did not involve any disruption of normal growth regulation and that the cells retained their DNA repair capabilities, indicating a natural, controlled pathway of renewal.
The researchers discovered that this process was driven by the natural activation of telomerase and PGC1α, allowing cells to reset their biological clocks by extending chromosomal ends and regenerating mitochondria.
Why It Matters
Beef is the most resource-intensive form of agriculture, responsible for deforestation, water depletion, and a significant share of global greenhouse gas emissions. Cultivated meat, grown from animal cells rather than livestock, has long been touted as a solution. However, challenges related to cost and safety have slowed progress, especially for cultivated beef.
Science, Patience, and Serendipity
According to Prof. Nahmias, “Months stretched into years, and perseverance replaced certainty. Then, after over 400 silent days, colonies suddenly appeared—a true eureka moment that overturned what we thought we knew about bovine cells.”
The discovery also sheds light on a long-standing biological puzzle known as Peto’s paradox—the observation that large animals rarely develop uncontrolled cell growth despite having far more cells. The team’s work suggests that the same natural defenses that protect large animals may render their cells more resistant to renewal until time and evolutionary forces allow adaptation.
The researchers are now investigating whether the same natural renewal process can occur in other mammals and whether these cells can be developed into muscle and fat tissues suitable for cultivated meat production.
“This work adds valuable new insights to the rapidly expanding knowledge base supporting cultivated meat development. Spontaneous immortalization attempts often fail because researchers simply abandon the process when cell growth slows,” Dr. Elliot Swartz, Sr. Principal Scientist for Cultivated Meat, The Good Food Institute. This study demonstrates for the first time that bovine cells can be spontaneously immortalized, marking an exciting advance. By detailing the sequence of events that occur during cell line development, it provides a roadmap for non-GM approaches to be used for commercial cultivated meat production across the full range of animal species used in food production.
The research paper, titled “Spontaneous immortalization of bovine fibroblasts following long-term expansion offers a non-transformed cell source for cultivated beef,” is now available in Nature Food here.
Researchers:
Laura Pasitka1, Merav Cohen1,2, Shaun Regenbaum1, Avner Ehrlich1, Boaz Gildor3, Ariel Gold3, Yaakov Nahmias1,2,3
Institutions:
- Alexander Grass Center for Bioengineering, The Hebrew University of Jerusalem
- Alexander Silberman Institute of Life Sciences, The Hebrew University of Jerusalem
- Believer Meats; Rehovot